FDA Decision Postmortem: Praecis
If
you have any comments, questions, or thoughts on this article or on anything to
do on biotechnology or medical technology feel free to email me at prugg@tradingmarkets.com.
On
June 12, the FDA Advisory Committee on oncologic drugs rejected the
new drug application of a new treatment for advanced prostate cancer, a growing
market. The drug, Plenaxis, was
co-developed by Praecis Pharmaceuticals
(
PRCS |
Quote |
Chart |
News |
PowerRating) and Amgen
(
AMGN |
Quote |
Chart |
News |
PowerRating), both
of which took a hit in the financial markets.
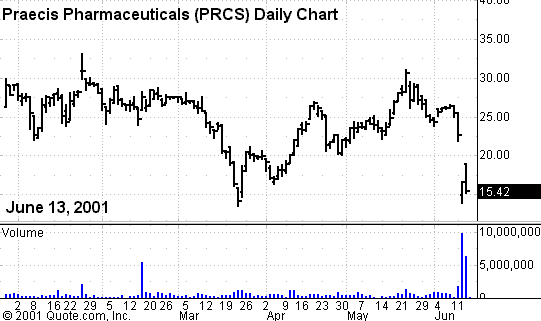
Praecis
Pharmaceuticals took the brunt of the bad news, with its stock price plummeting
27% on the rejection. It took the brunt
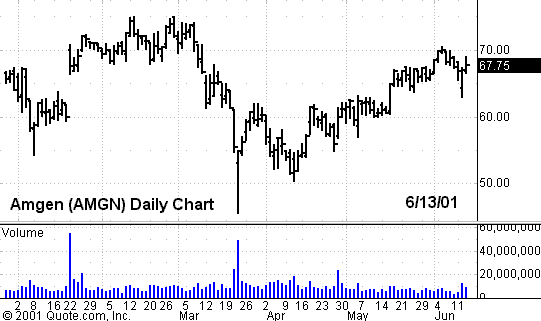
because this would have been its first revenue-generating product. Amgen had
less to lose. This episode is just
another example of how much absolute power the FDA possesses over short- and
long-term equity movement.
In this and other
instances, the FDA gives true meaning to the saying, “It ain’t over until
the fat lady sings.†The FDA is
truly the fat lady when it comes to placing the final blessing on the future
growth potential of any biotechnology or pharmaceutical company.
Unfortunately, on Tuesday Praecis Pharmaceuticals and Amgen were in the
audience when the FDA sang.
This decision took
many by surprise, given the recent release of positive Phase III clinical data
at the June 2 meeting of the American Urological Association.
The data looked good in regard to showing efficacy of the drug to treat
advanced prostate cancer, in comparison to existing treatment.
The safety profile of the drug in the final phase studies also looked
acceptable. Many, including myself,
expected this drug to be approved, give a boost to the stock price, and add to
the company’s long-term revenue projections.
So, what was the
problem with the application and why did the FDA decide against approval?
At this point in time, only the FDA has the answers to these questions.
It is possible the FDA Advisory Committee saw something in the safety
profile of this drug they did not like. Drug
safety and toxicity are very sensitive issues nowadays with the FDA, given some
highly publicized recalls in the past year due to unexpected side effects.
This organization
will not hesitate to reject a new drug application if the data does not
conclusively address safety issues. It is
also possible the FDA felt the new drug did not adequately make its efficacy
case against the current approved drug (Lupron) on the market today.
Lupron does work well in treating advanced prostate cancer and it
can be an uphill battle for a young company to get a new drug approved, unless
it convincingly trounces the existing competition in clinical trials.
Was it possible for
investors to see this coming, based on the stock’s price activity weeks or
days prior to the meeting? The charts
usually have a story to tell and can help in finding an answer.
Normally, positive Phase III clinical data presented at a major medical
meeting cause a nice bounce in the stock price, right before and after the news
announcement. In this case, the bounce
was not evident. Was this an ominous
sign of things to come?
How should investors
now look at Praecis Pharmaceuticals in the long term? Interestingly enough,
Credit Suisse First Boston yesterday
upgraded the company to a strong buy based on its current valuation. The
company does expect to address the concerns of the FDA once it meets with the Advisory
Committee and expects approval of the drug sometime next year.
However, this is the
standard spin all companies put forth after a major insult and only time will
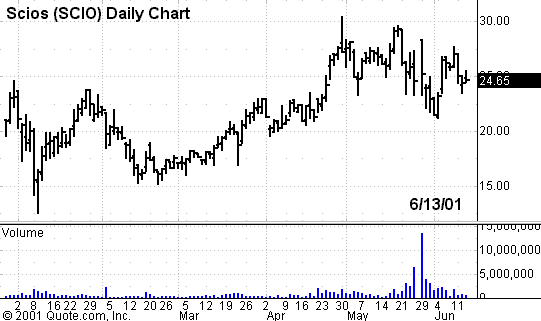
tell. Interestingly enough, a new drug just approved for congestive heart
failure by Scios
(
SCIO |
Quote |
Chart |
News |
PowerRating) followed a similar path to ultimate FDA approval. Scios’ new first drug was originally rejected by the FDA two years ago.
The stock price fell to $3/share at the time of the rejection. The company met
with the FDA, accumulated more data, and won approval on May 25, 2001. The stock
price closed at $24.65 yesterday.
What does this event
tell me about the FDA? It tells me
the FDA is Greenspan-esque in its ability to camouflage its thinking, right up
to the time of a major decision. It also
tells me, in Greenspan-esque fashion, that when the FDA sings, everyone listens.
