The FDA’s Helping Hand To Investors
If you have any comments, questions, or
thoughts on this article or on anything to do on biotechnology or medical
technology feel free to email me at prugg@tradingmarkets.com.
On June 28, we saw the positive influence the FDA can have on a company’s stock
price and future drug development and how investors can take advantage of it.
Several companies filed New Drug Applications (NDAs) with the FDA on June 28 for their lead drugs. The filing of a new drug application or NDA is the first
tangible step a company takes in the process of getting a new drug approved by
the FDA.Â
The application is made after several large Phase III clinical trials
have been completed and show the new drug to be safe and effective. Once the NDA
is filed, it may take several months for the FDA to accept the drug application.
There is a chance the FDA may reject application if the supporting
clinical data is not acceptable. Once
accepted, the FDA is telling the company, yes, you definitely have something
here.The next step in the
process to approval is a thorough review of the incoming clinical data. This
review could take up to a year before the FDA actually votes on whether or not
to approve the drug. However, if a drug
has been given “fast-track†status, then the average time to the definitive
vote can be four to six months. Fast-track
status means the FDA will expedite the processing of the designated application
because the drug is intended to treat a life-threatening condition, one that has
no current effective therapy. Â
Fast-track
status does not guarantee the drug will get approved.
However, its designation can have a very positive influence on the stock
price, when announced. During the time
the FDA is reviewing the application, the agency may ask for more clinical data
supporting the new drug’s effectiveness or, in the worst-case scenario, ask
the company to perform another large-scale clinical trial.Â
The
filing of an NDA is truly a seminal event in the life of a maturing
biotechnology company and investors do benefit from it if they are aware of the
actual date. The decision by the FDA to
accept the NDA application is also an event in the life of a biotech company
that excites investors and usually moves the stock price.
Ultimately, the big prize is drug approval and this event can be the
brass ring for investors. Â
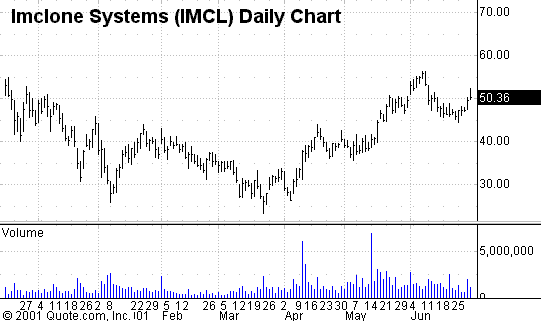
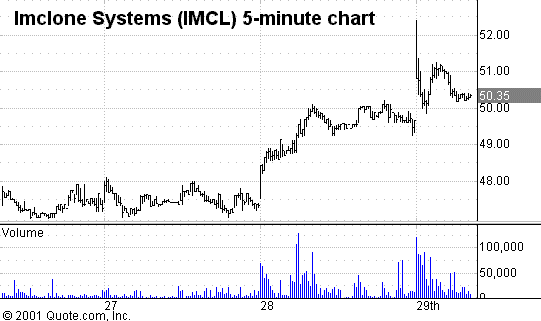
ImClone Systems
(
IMCL |
Quote |
Chart |
News |
PowerRating),
a leader in developing novel monoclonal antibody treatments for cancer, filed
what is called a “rolling†Biologic License Application (BLA) for approval
of its lead cancer drug, IMC-225. A
rolling designation only applies to drugs given the fast-track status and means
the company can submit data supporting the application periodically and not all
at once. The rolling designation allows
the company to constantly work with the FDA, obtaining feedback along the way,
and maximizing its opportunity for approval. A
biologic license application is synonymous with a new drug application.
Â
ImClone’s new drug has shown great promise as a new way to treat a
variety of cancers, especially colorectal cancer that is refractory to current
medical treatment. The company also has a
clinical pipeline of other novel drugs, including the development of vaccines to
treat cancer. Investors applauded the
application with a nice 5% move in the stock (see charts) and should keep their eye on this
company over the next six months.
Â
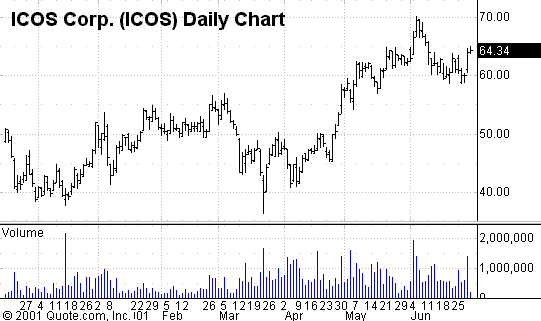
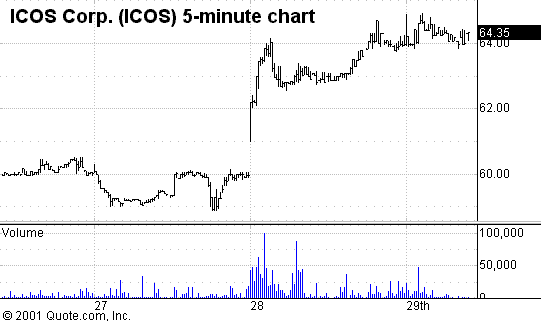
ICOS Corporation
(
ICOS |
Quote |
Chart |
News |
PowerRating),
along with Eli Lilly
(
LLY |
Quote |
Chart |
News |
PowerRating), also filed an NDA with the FDA for its drug to
treat erectile dysfunction. The company
recently presented very convincing Phase III data on its effectiveness and
safety in treating erectile dysfunction, or ED. The
ED market is huge and currently monopolized by Pfizer’s blockbuster Viagra. Â
However, ICOS has a formidable competitor in its new drug Cialis, because
it actually may work faster and longer than Viagra.
Again, the filing of the NDA rewarded investors by a 7% move in the stock
price (see charts).Â
ICOS also is developing a drug to treat another growing, untapped disease
market called sepsis that warrants investor attention in the long run.
Â
One last, but
definitely not least, company that was rewarded by the FDA yesterday was MGI
Pharma
(
MOGN |
Quote |
Chart |
News |
PowerRating).yes”> The drug irofulven is currently being tested in Phase III clinical
trials to treat a variety of cancers, including the deadly pancreatic, and the
company does not expect to file a new drug application (NDA) until all the data
is in. However, the FDA feels this drug
can fill an unmet need in the medical community and will expedite its review,
once the application is in. Again,
investors welcomed the designation, with a 14% increase in the stock price.Â
The above examples
are just some of the ways you, as an investor, can take advantage of the how the
FDA influences the potential growth of a company.
In July, I will give a two-part course on “Trading The FDA†and give
added insight to the ways investors can benefit from decisions made by the FDA.Until then,
I have been
investing/trading in medical and biotechnology stocks for years. The one common
theme is that this is a high risk/reward endeavor. When you are correct, many
times you are richly rewarded. When you are wrong, you can be badly punished.
Please keep this in mind and hedge/protect yourself appropriately when
trading/investing in these stocks.
