Searching For The Holy Grail In The Fight Against HIV/AIDS
Stock Alert: VaxGen
Today at noon Eastern Time, Feb. 11,
VaxGen
(
VXGN |
Quote |
Chart |
News |
PowerRating) will hold a
conference call discussing its earnings report for the quarter and year ended
Dec. 31, 2002. The question on every trader’s mind is not whether the company
made money last month or whether it will make money this year. The big question
is whether or not CEO Lance Gordon will shed some light on the much-anticipated
phase III clinical data on the company’s trademark AIDSVAX vaccine.
For the last several months, this company has been under
the medical and investor microscope because it has the lead on developing a
potential blockbuster vaccine to prevent the AIDS virus from spreading. The
company also has ongoing research programs in developing vaccines for smallpox
and anthrax. Several other companies have started programs to develop potential
vaccines to treat the AIDS. However, VaxGen is currently in the spotlight
because the company is farthest along. There are two ongoing phase III trials,
one in Thailand and one in the United States. VaxGen has stated that it will
report on the Thailand trial by the end of the first quarter of this year. The
US data will be reported later in the year.
Up until now, there has been no indication whatsoever what
the data will show. The results of these trials have been one of the best-kept
secrets in the biotech and medical industry, and for good reason. AIDS is a very
deadly, difficult to treat, and highly public disease. The first company to come
up with solid clinical data supporting a vaccine to prevent the spread of the
HIV virus will have found the Holy Grail in treating this disease.
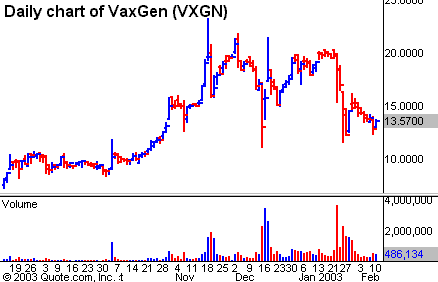
VaxGen has been in investors’ crosshairs for months and its
volatile trading volume has reflected this close scrutiny. Rumors circulated on
Jan. 23 that the clinical data was not very supportive of the efficacy of the
vaccine and the stock subsequently took a beating. At that time the company
reiterated that no data had been released yet and stuck by its first-quarter
revelation guns. Anticipation of this clinical data has been growing
exponentially. Unfortunately, expectation of solid clinical results showing
strong activity against the HIV virus has been shrinking. Several weeks ago,
expectation was that the vaccine worked 50% of the time. Now that seems to be
down to 30%. Whatever the number, I believe any percent will be better than
nothing if the vaccine proves to be safe. I can only describe how investors will
react to the release of this news when it does occur, with two words:
EXTREME
VOLATILITY.
Will CEO Gordon comment on the actual clinical data
in his conference call today at noon? Only he can answer this question and will
surely get his chance to a very captive audience.
My next piece, later this week, will talk about several
companies who are on the FDA center stage in March for new drug approval.
Good Luck,
Paul Ruggieri, MD, FACS
