Trading The FDA, Part II
If
you have any comments, questions, or thoughts on this article or on anything to
do on biotechnology or medical technology, feel free to email me at prugg@tradingmarkets.com.
As
I left off in Trading
The FDA Part I, the filing of a New
Drug Application
(NDA) is a sentinel event in the life of a biotechnology company.
It is an event that investors usually applaud with an immediate increase
in the stock price and with some follow-through several days afterward.
I see this time and time again, and gave several examples of a positive
market reaction to this event in my first lesson.
However, many times,
this enthusiasm is short-lived for a variety of reasons over the next 12 months.
Once a New Drug Application has been filed with the FDA, then the agency
needs to decide to accept it, based on the supporting clinical data.
As I mentioned earlier, the majority of new drug applications are
accepted and the period of time between NDA filing and acceptance is usually two
to four months.
Once the initial
wave of news reports has occurred announcing the NDA filing, investors seem to
have a short attention span. This short
attention span is often manifested by a slow, steady decline in the company’s
stock price until the next sentinal event. This
next sentinel event occurs when the FDA announces the acceptance of the New Drug
Application.However, without any
more potential positive news on the horizon, gravity slowly exerts a downward
momentum before the FDA acceptance announcement is made.
Any negative news during this time period can only add to the negative
decline. This scenario usually occurs
with small-cap, unprofitable companies looking to get their first drug approved.
I use Matrix Pharmaceuticals
(
MATX |
Quote |
Chart |
News |
PowerRating)
as an example of how these early events can act as entry and exit points for
investors watching a new drug make its way through the FDA decision process.Â
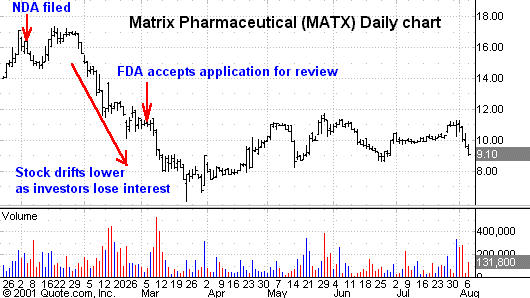
On Jan. 4, 2001,
Matrix Pharmaceuticals filed a New
Drug Application with the FDA for its new
inject able drug to treat refractory head and neck cancer.
This announcement served as an entry point for investors and the stock
responded accordingly. Again,
investors responded positively to the news. Unfortunately,
the two months in between were not kind to investors as the stock drifted lower,
waiting for the next big wave of news to come along.
Investors quickly lose interest once the news is out and wait for the
next opportunity for a stock price movement. However,
this choppy pattern does offer you an opportunity to plan early entry and exit
points.
What
Happens Once The NDA Application Is Accepted For Review?
This is where things
get very interesting. Before going on, I
need to make several points about the timeline ahead for many of these new
drugs. If the FDA has decided to
designate a new drug as “fast track†status, then it has an obligation to
make a final decision on the marketability of that drug within six months of
accepting its New Drug Application. If fast-track status
is not given, as is the case with most new drugs, then the FDA time line is
normally one year before an advisory committee meets to decide the drug’s
market fate. However, lately the FDA has
been taking longer (12 to 16 months total) from the time of New Drug Acceptance
to the actual decision on whether or not to approve the drug.Â
Now, once the FDA
decides to accept a New Drug Application, the best news a company can receive
prior to being notified of the actual advisory committee meeting date is NO
news. With all biotechnology companies,
at this stage in drug development, no news is good news.
However, this is not always the case and usually when the FDA speaks
before the time of the actual advisory committee meeting, the company in
question gets hurt.Many times, the FDA
will make an initial review of the clinical trial data supporting the
application and raise questions about a drug’s efficacy and safety.
Unfortunately, these questions can only delay the final outcome of the
New Drug Application, delay the market arrival of the drug, delay projected
potential revenues and make investors very nervous.
At this point, the stock price often reflects how nervous investors get.
United Therapeutics
(
UTHR |
Quote |
Chart |
News |
PowerRating) is a
prime example of how any delay in a New Drug Application can hurt a company’s
stock price.Â
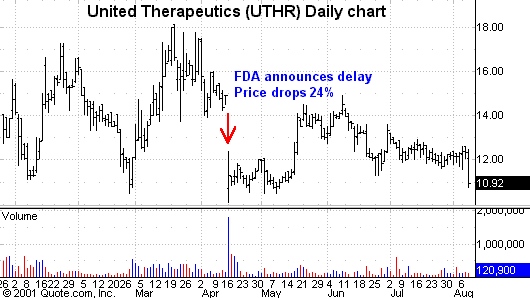
On April 16, the FDA
announced a delay in the processing of the company’s NDA for its drug to treat
pulmonary artery hypertension.Â
In addition to
smaller biotechnology companies feeling the pain of a delay, the more
established, profitable companies can also take a hit, if a high expectation drug
is delayed. Genentech
(
DNA |
Quote |
Chart |
News |
PowerRating) is a prime example of this.Â
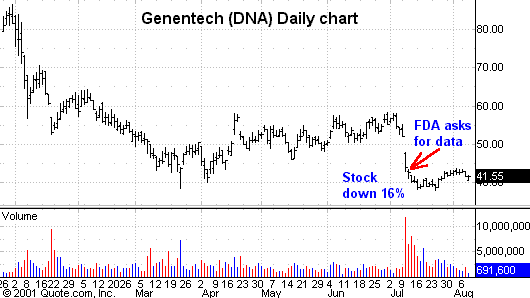
On July 11, 2001,
the FDA asked for more clinical data to support the safety of Genentech/Tanox’s new drug to
treat asthma. This new drug was aimed at
a very large market and has the potential to generate over a $1 billion in
potential revenue if approved. Unfortunately,
as big as the potential revenue stream was for Genentech, the hit
the company took because of this delay was even bigger.
On the day of the FDA news, the stock price declined 16%.
Even more striking was the negative reaction aimed at the company co-developing
the drug, Tanox
(
TNOX |
Quote |
Chart |
News |
PowerRating).
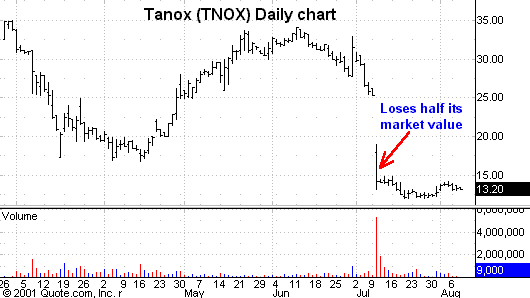
Along with
Genentech’s decline, Tanox lost close to half its market value that day on the
news that would result in a delay in the asthma drug coming to market. Again, the smaller-cap companies looking to gain market share with their
first new product get hurt the most if the FDA brings negative news.Â
Can
investors anticipate these potential land-mine delays before the FDA makes a
final decision on a new drug?Â
The short
answer is no, unless you have access to the clinical data reviewed by the FDA.
However, there is a way to begin to understand how the FDA is thinking
when it decides to delay the final action on a New Drug Application.
Any new drug aimed at a large disease market such as cancer, diabetes,
heart disease, stroke, Alzheimer’s and the list can go on, has high
expectations and can lead to some nice gains.Â
Unfortunately, if the FDA expresses any doubt about the drug before it
has a chance to decide its fate, then the downside drop can be steep.
Be vigilant of new drugs aimed at treating a childhood diseases.
Children hold a special place in the heart of the FDA and the clinical
data presented with the New Drug Application better be extremely safe and
pristine.Â
Beware of new drugs
that are called a “new class†of treatment.
New class drugs invite extra scrutiny by the FDA with regard to safety
concerns, especially if there already are approved treatments for the disease in
question.Â
Also beware of companies
casting a wide application net for their new drugs, since this also will invite
much scrutiny from the FDA before the meeting date is set.
Often a company will list several disease conditions that its new drug
could potentially treat.
Unfortunately,
Genentech and Tanox’s new asthma drug encompassed many of the points made
above, and thus invited much scrutiny from the FDA.
The drug was aimed at treating both adults and children with asthma and
allergies. It was also a new class of
drug, a monoclonal antibody, different from anything already being used by
doctors today. The end result was that the FDA
got squeamish and requested more data covering the safety of the drug.
Genentech and Tanox now have amended their application to include only
adults with asthma.New
Drug Application Accepted For Review, What Next?
Once a company’s
application has been accepted for review, there is no guarantee the drug will
get approved for use. It can take up to
18 months (usually 12) for the FDA to meet and decide if the drug is acceptable.
As I mentioned earlier, “fast track†status shortens this time to six
months. Like the
aforementioned sentinel events, it offers you another opportunity to enter and
exit the stock. This event is the
announcement of the all-important FDA advisory committee meeting, the meeting
that will ultimately determine the fate of the drug and, many times, the company
presenting it.
Good luck with your
trading. I look forward to seeing you at TradingMarkets
2001.
I have been
investing/trading in medical and biotechnology stocks for years. The one common
theme is that this is a high risk/reward endeavor. When you are correct, many
times you are richly rewarded. When you are wrong, you can be badly punished.
Please keep this in mind and hedge/protect yourself appropriately when
trading/investing in these stocks.
For The Best Trading
Books, Video Courses and Software To Improve Your Trading
